Practically, what are we supposed to do with all of these isotopes? Scientists need to know how much each element weighs so that they can make accurate calculations, so what number do they use when they want to figure out how much a gram of carbon weighs?
The atomic weight of an element is the weighted average of the relative atomic masses of each of the element's stable isotopes or long-lived radioisotopes, taking into account each of their relative abundances. Radioisotopes generally are not used in calculations of atomic weight because the relative abundance of a radioisotope will change as time passes. However, if a radioisotope has a very long half-life, it is considered to be long-lived, and therefore is included in the calculation if its relative abundance is large enough to contribute. The equation used to calculate atomic weight for an element having two stable isotopes (or long-lived radioisotopes) is:
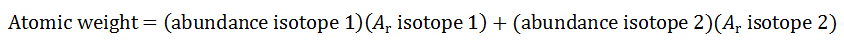 |
As you have already learned, the relative heights of the peaks in a mass spectrum are determined by the relative abundances of the different isotopes of an element. From these relative intensities, we can obtain the abundances of the various isotopes, which in turn can be used to calculate the atomic weight of an element.
Being able to calculate the atomic weight of an element is important, not only to chemists, but also to many of the disciplines which use chemicals. For example, pharmacists need to be able to relate the weight of each medicine dosage to the chemical amount of the drug within each dose. Drugs are developed to be effective when a specific chemical amount of that drug is consumed, but given their size, it is impossible to prescribe doses for patients in the terms of counting molecules. Medical professionals use atomic weight and other data which helps define how much an element or molecule should weigh. With terms like molar mass, which defines how much one mole of a substance weighs, pharmacists and doctors can accurately weigh out medication and know exactly how much of an chemical substance will be active in treating a patient.