Why should we care?
These intervals are not only present on the IUPAC Periodic Table of the Elements and Isotopes, but are listed on the most recent version of the IUPAC Periodic Table of the Elements. In order to interpret the data on the Periodic Table of the Elements, you will need to understand these intervals.
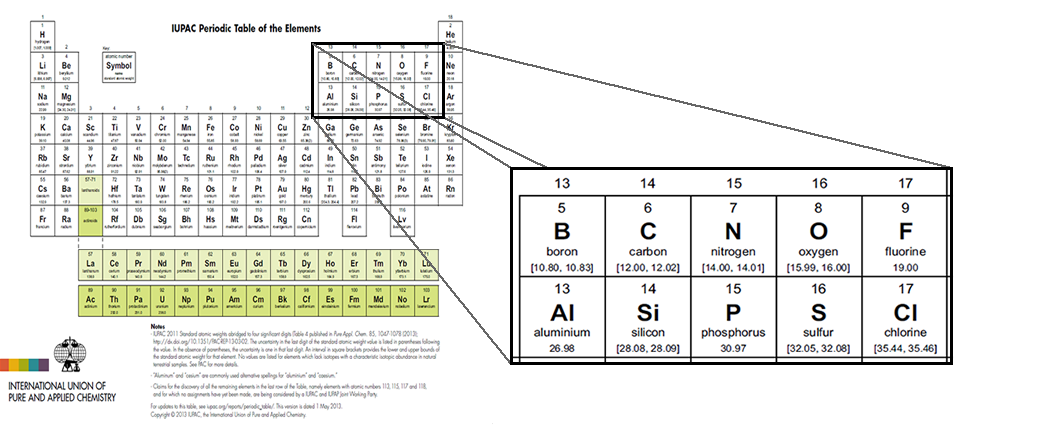
Your turn
New periodic tables show an interval of atomic weights for some elements rather than a single value. Why has this change been made?
- Improved mass measurements reveal new uncertainty
- Mass is constantly turned into energy via Einstein's equation: E=mc2
- Different samples of an element may have slightly different isotopic abundances
- New forms of measurement also take the masses of radioactive isotopes into account
Click here to show answer
The correct answer is C.
Why hasn't this change been made for all elements?
Click here to show answer
Right now the pink isotope category is the only one with intervals. Eventually, as IUPAC gathers more evidence for their isotopic abundance variability, some of the elements in the yellow isotope category will be moved to the pink category and be listed with atomic weight intervals. Isotopes in the blue and white categories will never be listed as having interval weights. The blue isotope category represents elements with only one isotope used to determine atomic weight, so the weight never changes with isotopic abundances. The white isotope category has no standard atomic weight because all isotopes are radioactive.