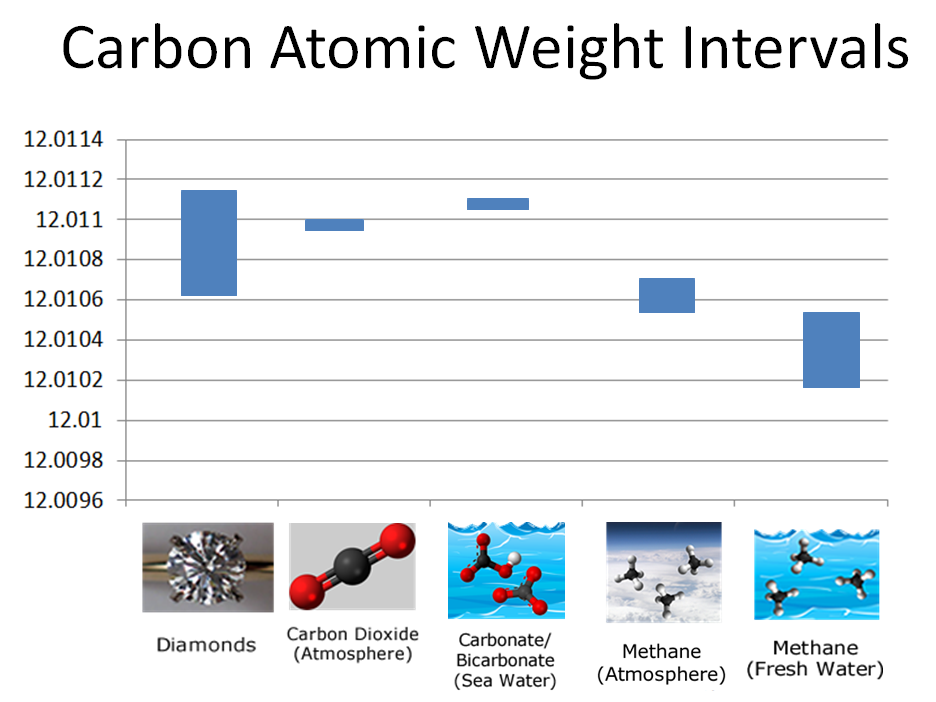
As noted previously, atomic weight intervals of atoms vary depending on their source. Examine these atomic weight interval tables for hydrogen and carbon. Notice that the atomic weight of atoms can vary from compound to compound (such as hydrogen atoms from ethanol to methane) or within the same compound, but from different sources (such as carbon atoms in methane from air or from fresh water).
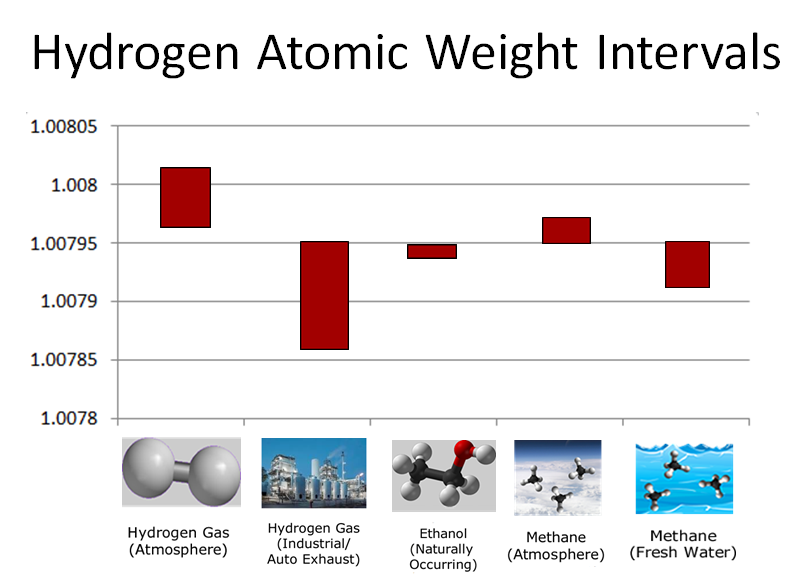

1) Look at the five different sources of hydrogen atoms listed on the above table.
Which has the highest possible atomic weight?
2) If you were given a sample containing carbon atoms, whose atomic weight was measured to be 12.0106 and you knew it had to be from one of the sources listed in the table above, which source would be most likely?
3) Consider the atomic weight intervals for both hydrogen and carbon atoms from methane obtained from different sources listed above. Which combination do you predict would produce the lower molecular weight interval for methane?
- Hydrogen atoms from "Methane (Atmosphere)" + carbon atoms from "Methane (Atmosphere)"
- Hydrogen atoms from "Methane (Fresh Water) + carbon atoms from "Methane (Fresh Water)"
Hydrogen atoms in "Methane (Fresh Water)" molecules have a lower atomic weight than those from "Methane (Atmosphere)". Likewise, carbon atoms in "Methane (Fresh Water)" have a lower atomic weight than in "Methane (Atmosphere)". Therefore, we would predict that the combination of hydrogen atoms from freshwater methane and carbon atoms from freshwater methane would produce a lower molecular weight for methane.