Oxygen's two most abundant stable isotopes are oxygen-18 and oxygen-16. A water molecule contains a single oxygen atom, which may be either isotope. For instance, an oxygen-18 isotope would form H218O, and an oxygen-16 isotope would form H216O. H218O is the isotopically heavier form of water, and H216O is the isotopically lighter form of water. (Conventionally, we only use the term "heavy water" to refer to a water molecule that contains one or two atoms of deuterium (2H)).
When we drink water, the atoms present in the water molecules are deposited in our bones and teeth. This exchange continues in the bones throughout adulthood, but in the teeth the enamel is effectively 'sealed' off once development is complete. This means that the enamel in teeth provides an isotopic record of aspects of childhood life while the bones record the specific isotope ratios from water consumed as an adult. In this way, the isotopic signature, the oxygen isotope ratio in the water we drink, is recorded in our body. We can study Ötzi's bones and teeth to discover how much oxygen-18 was present in the water molecules he consumed compared to the amount of oxygen-16.
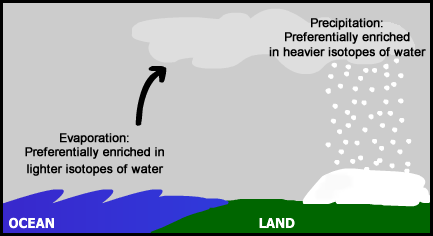
This can help us to locate where Ötzi lived, because as a water source position changes, commonly so does the ratio of oxygen-18 to oxygen-16 in the water molecules. Recall that the different number of neutrons in the oxygen atoms result in water molecules with different abundances of oxygen isotopes. This causes variation in the molar masses of water molecules. As water vapour in the air travels over land from the ocean, some of it will precipitate as liquid or solid water (ice), depending on the temperature. Because H218O is heavier, it preferentially precipitates over H216O. Precipitation in areas that are further inland and at higher elevations are preferentially depleted in H218O relative to H216O. This means that measuring the ratio of the number of oxygen-18 and oxygen-16 atoms in Ötzi's bones and teeth can commonly provide information on how far inland or at what relative altitudes his water sources were. Because the measurement of the oxygen-isotope ratios is a differential measurement on a mass spectrometer (or laser absorption spectrometer), we express these as δ18O, which is calculated from the 18O to 16O ratio in a sample and a standard 18O to 16O ratio.
If you have never seen this (δ) symbol before, don't worry. It's a lower case delta; you may have encountered the upper case delta (Δ) before, and might know that it usually indicates a change. When we use δ18O, we are expressing an oxygen-isotope ratio in a sample relative to that in a standard, or how much our experimental isotope ratio values differ from the standard ratio.