You are given a sample of water from the Alps where Ötzi was found. You have already analyzed the mass spectrometry data and have determined the ratio of 18O to 16O is 1.972 x 10-3.
The 18O to 16O standard reference ratio we use is called the Vienna Standard Mean Ocean Water (VSMOW) and has the value: 2.005 x 10-3. Calculate the δ18O using the following equation.
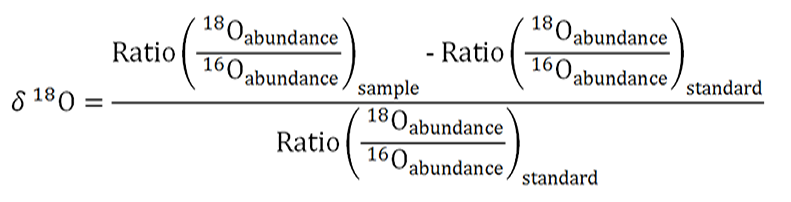
(Where ‰ is the symbol for per mil and equals 0.001)
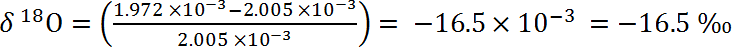
The concentration of 18O in water molecules decreases as we take samples from sources further inland. Order the following δ18O values from most coastal source to furthest inland source.
- -13.8×10-3 = -13.8 ‰
- -16.5×10-3 = -16.5 ‰
- -9.7×10-3 = -9.7 ‰
If the concentration of 18O in water decreases as we sample from sources further inland, then using the equation from the previous question, we can calculate that δ18O values also decrease as we sample from sources futher inland. Remember that these values are negative, so -9.7 is a larger value than -16.5.