Test Your Knowledge
Key Idea 6: Ötzi the Iceman
29) In the investigation of Ötzi the Iceman, the variation in isotopic abundances was used to analyze:
- The kinds of meals he had eaten just prior to his death
- Hypotheses about medicine at the time
- Where he may have lived
- The kind of ink used in the tattoos on his arthritic joints
30) Strontium—87 was one of the radiogenic isotopes analyzed in the investigation of Ötzi. What does it mean for an isotope to be radiogenic?
31) You learned that strontium and lead isotopes were analyzed in Ötzi’s bones and teeth. How did the isotopes of these elements end up his bones and teeth?
32) Taking it Further: You were so inspired by learning about the story of Ötzi that you decided you become a forensic archaeologist! Your first case is to help locate where a second frozen mummy named Akash was originally from. To discover the abundance of the isotopes in the water molecules in Akash’s teeth, you plan to use the technique of mass spectrometry. But first, it is important for you to learn about how molecular information is shown on mass spectra. To test your knowledge, you are given a sample of water molecules that have a variety of isotopic combinations. Hydrogen has two relatively abundant stable isotopes: hydrogen-1 and hydrogen-2 (deuterium), and oxygen has three relatively abundant stable isotopes: oxygen-16, oxygen-17 and oxygen-18. The isotopic combinations included in a few representative molecules from the sample are shown below. At what mass/charge ratio do you expect to see peaks in the mass spectrum? (Hint: Pay attention to the difference in molecular masses of the water molecules. Remember: The masses of the constituent isotopes will determine the masses of the different peaks for water molecules on the mass spectrum.)
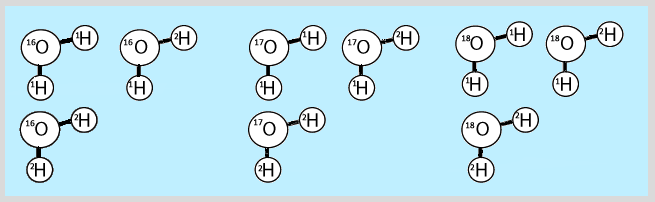
33) The hunt for Akash's place of residence has been narrowed down to two areas: one of them is inland and other is near a body of water. In which of these two locations would you expect the water molecules found in a frozen mummy to be enriched more in oxygen-18 atoms? How could you use this information to give clues as to which location Akash was from?
34) If the relative abundance of oxygen-18 atoms is higher in his teeth than in his bones, did Akash likely move further inland or closer toward the shore during his lifetime?